Economic benefits of the continuous chromatography process MCSGP
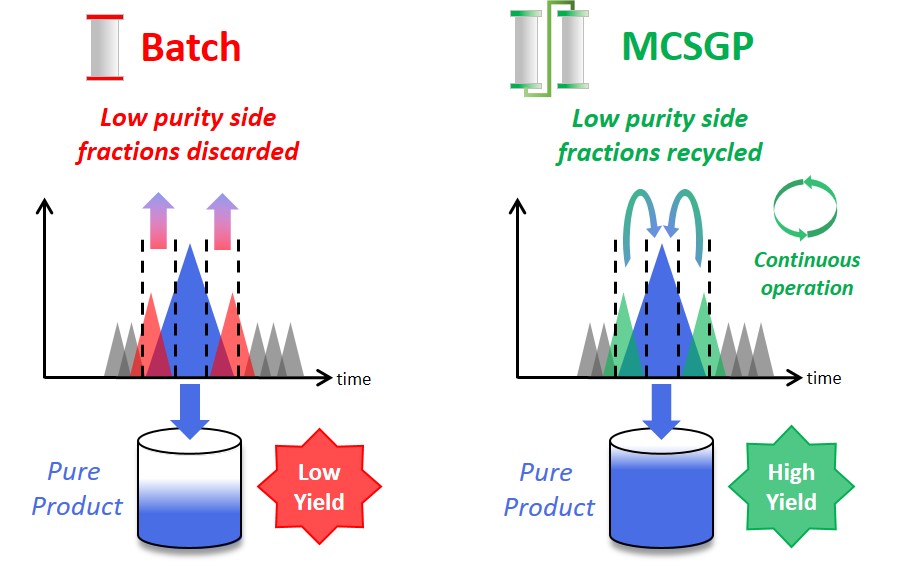
MCSGP is a chromatographic method operated continuously using two columns, compared to the batch mode of single-column chromatography (see above). Much of the basic principle, however, remains the same. In a chromatographic process, the impure feed mixture is separated, giving fractions with pure product, fractions with a mixture of impurities and product, and fractions containing only impurities. Pure product fractions are collected and impure product-containing side fractions are discarded or re-processed. If product containing side fractions are discarded, valuable product is lost. Reprocessing the side fractions accumulates impurities and applicability is thus limited. Moreover, reprocessing reduces overall productivity. MCSGPcan isolate most of the product included in the starting material by internally recycling product-containing side fractions and repeatedly separating impurities and pure product. The recycling is done automatically, without the need for off-line analysis. The impure side fractions are transferred from one chromatographic column to another. Fresh feed material is added every cycle. This ensures continuous operation and leads to a product with high yield without compromising purity.
Purification of MCSGP with Contichrom technology
MCSGP offers significant advantages over single-column chromatography in the purification of oligonucleotides. The advantages include:
- Oligonucleotide yields were increased by at least 50%, leading to over 90% recovery at 92% purity (target purity was >91%)
- For single-column chromatography, the recovery was 60% at 92% purity
- Increased yield allows massive scale-down of oligonucleotide synthesis upstream to obtain required oligo amounts
- The productivity was increased 2-fold compared to the Benchmark run
- A massive reduction in the number of samples for analytical characterization, especially if re-chromatography of waste fractions is carried out. (Only one sample is generated per MCSGP cycle for two feed injections, while multiple fractions per injection are generated in single-column chromatography)
- The high yield of MCSGP makes re-chromatography obsolete
- All other process parameters were comparable to the batch runs
The increase in productivity by MCSGP allows multiple manufacturing options:
- The same target amount can be produced within the same time with smaller columns
- A specified target amount can be produced in a shorter period of time with columns of the same size
- More target compound can be produced per total column volume in the same time





